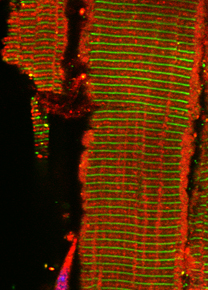
Titin (also called connectin) is the largest single polypeptide. It is modularly composed of immunoglobulin-like (IgG-like) and fibronectin III (Fn3-like) domains, which are arranged in patterns specific for the sarcomeric substructures: the Z-disc, I-band, A-band and M-band.
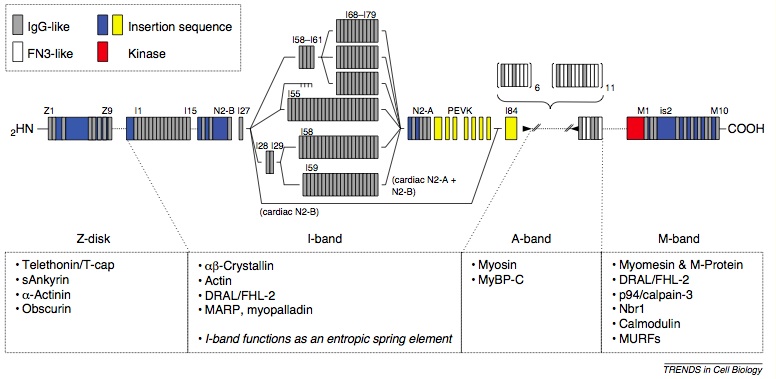 Model of titin domain structure and binding sites for sarcomeric and extra-sarcomeric proteins.
Model of titin domain structure and binding sites for sarcomeric and extra-sarcomeric proteins.
Published in Trends in Cell Biology.
Follow the link for access to the full manuscript (subscription may be required).
|
Molecular Functions
Titin fulfils a multitude of roles for the generation, function and maintenance of skeletal and heart muscles:
- Titin serves as a blueprint for the buildup of the myofibrils, as it contains binding sites for most sarcomeric components.
- Titin acts as tuneable passive spring element through its unstructured regions (N2A, N2B and PEVK). Titin's action can be fine-tuned through phosphorylation and expression of alternate splice variants.
- Titin assists in muscle specific signaling through signalling adaptors like proteins of the FHL or MARP family, or directly through its kinase domain. Biological functions of titin signalling are mostly related to biomechanics stretch sensing or signaling.
- Titin helps preserve the sarcomere by harbouring binding sites for chaperones (alphaB Crystallin), proteases and ubiquitin E3-ligases (e.g. MuRF proteins).
Muscle specific signalling and fine-tuning of muscle compliance
Titin harbours many mechano-responsive and mechano-sensitive elements. Some are located at the Z-disc, I-band and M-band of the sarcomere. Some of these elements may be able to sense and react to mechanical strain directly, like titin's stretch-sensitive kinase domain (read the manuscript here).
The I-band region of titin is thought to harbour several mechano-sensitive/reactive elements. In three recent manuscript we investigated the molecular functions of four-and-a-half lim domain (FHL) and muscle ankyrin repeat proteins (MARPs). Both protein families bind to the unstructured N2B and N2A regions of titin's I-band region. They serve as adaptor proteins for other signaling proteins (like protein kinases), and are able to influence titin compliance through the regulation of access to phosphorylation sites within titin.
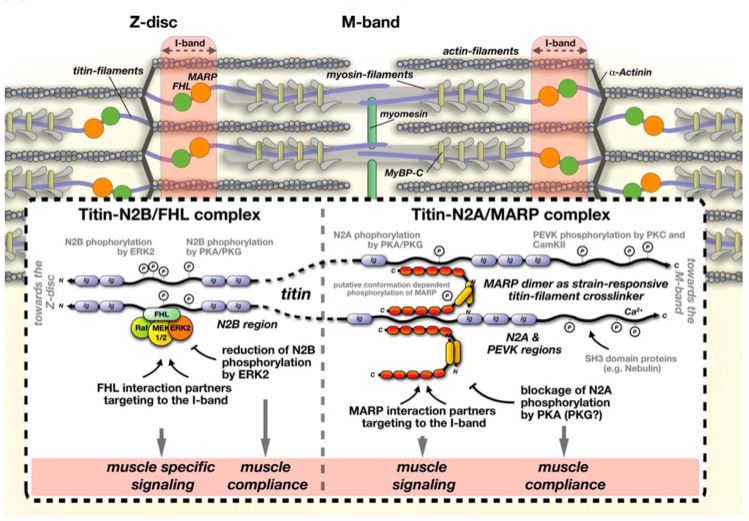 Titin-linked mechano-responsive complexes at the I-band.
Titin-linked mechano-responsive complexes at the I-band.
Published in Anatomical Record. Follow the link for access to the full manuscript.
|
New research from our and other lab as further elucidated the role that MARPs play for modulation of muscle compliance. Specifically, we and others discovered the formation of a trimeric complex consisting of the the MARP family member cardiac ankyrin repeat domain continaing protein 1 (CARP1), the 'elastic' filament titin, and the 'thin' actin filaments. A research article describing this trimeric complex and a new review article explaining the multifaceted roles of the titin N2B and N2A regions play as biomechanical and metabolic signaling hubs in cross-striated muscles summarize our most recent findings.
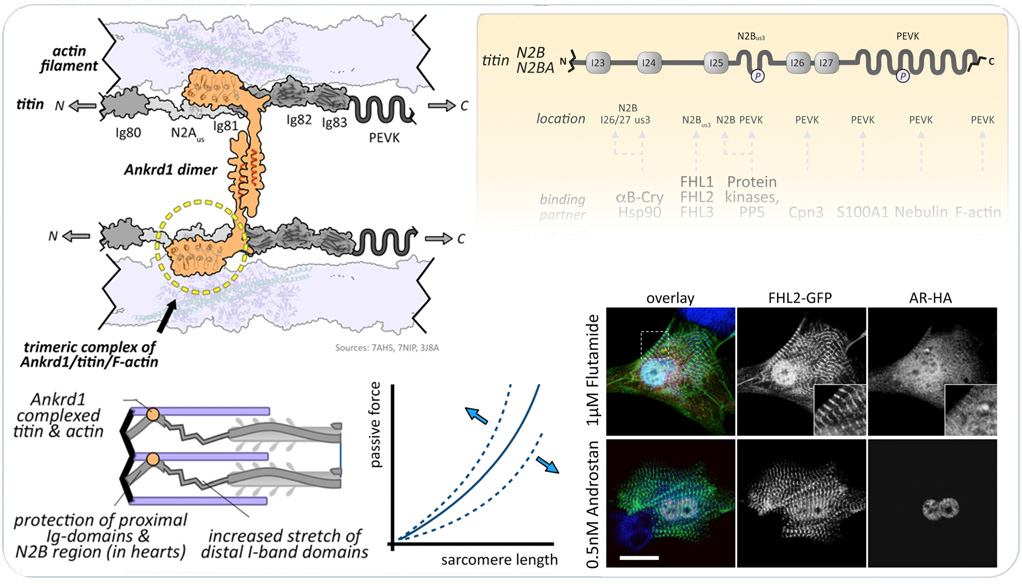 The titin N2B and N2A regions as biomechanical and metabolic signaling hubs
The titin N2B and N2A regions as biomechanical and metabolic signaling hubs
|
Disease links
Because of its enormous size, and its importance for muscle development and function, titin may be particularly susceptible for disease causing mutations. Titin mutations may cause different types of skeletal and cardiac myopathies depending on the type of mutation and the occurrence of 'secondary hits' (either two mutations in titin or a combination of a titin mutation and a mutation in a titin binding partner that are by themselves harmless, but in combination disease causing).
We try to elucidate the mechanisms of how titin exerts its many functions for skeletal and cardiac muscles on a molecular level. Of particular interest are titin causing mutations are their effects on titin binding-partners, as well as its muscle specific signalling function.
Future Directions
We are currently investigating the biological role that titin and its binding partners proteins play for dilated cardiomyopathy and limb-girdle muscular dystrophy disease development. Of particular interest are possible links between titin and its binding partners that modulate muscle specific signaling and muscle compliance.
Collaborators on the obscurin protein project are:
Dr. Elisabeth Ehler, King's College London, UK
Dr. Katja Gehmlich, Oxford University, UK
Dr. Olga Mayans, University of Konstanz, Germany
Dr. Jennifer Fleming, University of Konstanz, Germany
Related manuscripts
- The titin N2B and N2A regions: biomechanical and metabolic signaling hubs in cross-striated muscles. Biophys Rev. (2021). doi:10.1007/s12551-021-00836-3. read at Biophysical Reviews.
- The N2A region of titin has a unique structural configuration. J Gen Physiol. 2021. DOI:10.1085/jgp.202012766. read at the Journal of General Physiology
- Molecular characterisation of titin N2A and its binding of CARP reveals a titin/actin cross-linking mechanism. JMB. 2021. DOI: 10.1016/j.jmb.2021.166901. read at the Journal of Molecular Biology
- Probing Muscle Ankyrin-Repeat Protein (MARP) Structure and Function. Anat Rec. 2014 Aug;14. DOI: 10.1002/ar.22968. read at the publisher
- Reply: Hereditary myopathy with early respiratory failure is caused by mutations in the titin FN3 119 domain. Lange S, Edstrom L, Udd B, Gautel M. Brain. 2014 Feb 27. PMID: 24578547
- A novel mechanism involving four-and-a-half LIM domain protein-1 and extracellular signal-regulated kinase-2 regulates titin phosphorylation and mechanics. askin A, Lange S, Banares K, Lyon RC, Zieseniss A, Lee LK, Yamazaki KG, Granzier HL, Gregorio CC, McCulloch AD, Omens JH, Sheikh F. J Biol Chem. 2012 Aug 24;287(35):29273-84. PMID: 22778266
- An FHL1-containing complex within the cardiomyocyte sarcomere mediates hypertrophic biomechanical stress responses in mice. Sheikh F, Raskin A, Chu PH, Lange S, Domenighetti AA, Zheng M, Liang X, Zhang T, Yajima T, Gu Y, Dalton ND, Mahata SK, Dorn GW 2nd, Brown JH, Peterson KL, Omens JH, McCulloch AD, Chen J. J Clin Invest. 2008 Dec;118(12):3870-80. PMID: 19033658
- Interactions with titin and myomesin target obscurin and obscurin-like 1 to the M-band: implications for hereditary myopathies. Fukuzawa A, Lange S, Holt M, Vihola A, Carmignac V, Ferreiro A, Udd B, Gautel M. J Cell Sci. 2008 Jun 1;121(Pt 11):1841-51. PMID: 18477606
- Rigid conformation of an immunoglobulin domain tandem repeat in the A-band of the elastic muscle protein titin. Mueller S, Lange S, Gautel M, Wilmanns M. J Mol Biol. 2007 Aug 10;371(2):469-80. PMID: 17574571
- Evidence for a dimeric assembly of two titin/telethonin complexes induced by the telethonin C-terminus. Pinotsis N, Petoukhov M, Lange S, Svergun D, Zou P, Gautel M, Wilmanns M. J Struct Biol. 2006 Aug;155(2):239-50. PMID: 16713295
- Palindromic assembly of the giant muscle protein titin in the sarcomeric Z-disk. Zou P, Pinotsis N, Lange S, Song YH, Popov A, Mavridis I, Mayans OM, Gautel M, Wilmanns M. Nature. 2006 Jan 12;439(7073):229-33. PMID: 16407954
Work from this manuscript was featured in the RCSB Protein Data Bank's Molecule of the Month series.
- The kinase domain of titin controls muscle gene expression and protein turnover. Lange S, Xiang F, Yakovenko A, Vihola A, Hackman P, Rostkova E, Kristensen J, Brandmeier B, Franzen G, Hedberg B, Gunnarsson LG, Hughes SM, Marchand S, Sejersen T, Richard I, Edstroem L, Ehler E, Udd B, Gautel M. Science. 2005 Jun 10;308(5728):1599-603. PMID: 15802564
- Subcellular targeting of metabolic enzymes to titin in heart muscle may be mediated by DRAL/FHL-2. Lange S, Auerbach D, McLoughlin P, Perriard E, Schaefer BW, Perriard JC, Ehler E. J Cell Sci. 2002 Dec 15;115(Pt 24):4925-36. PMID: 12432079
|