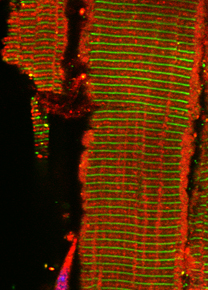
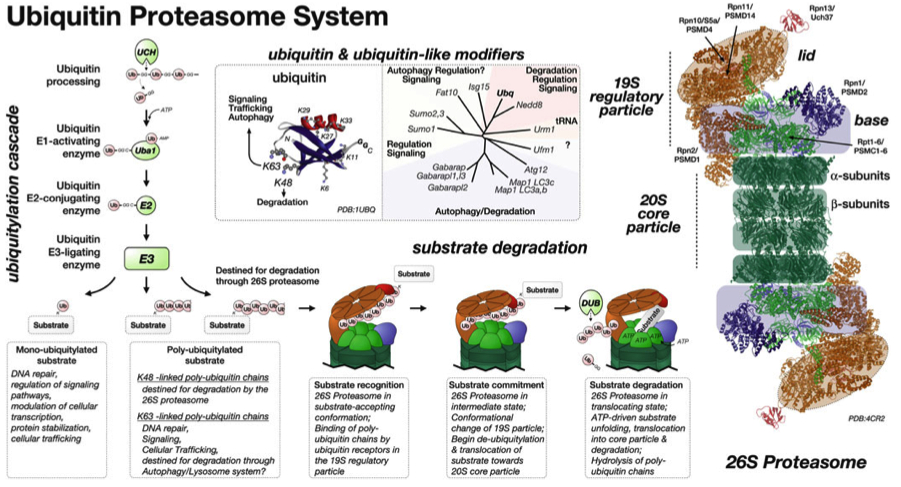
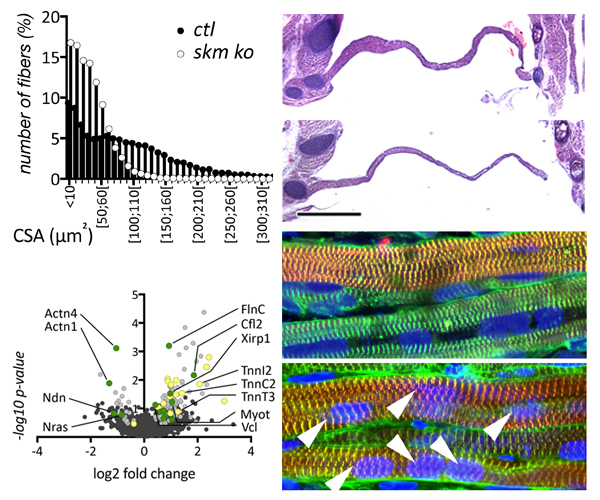
In our new manuscript, we outlined the role that this E3-ligase function plays for the development of nemaline myopathy in a murine model of the disease. Using mice that are deficient of Cullin-3, we define a novel molecular pathomechanism that may be at play in patients suffering from mutations in KBTBD13. The manuscript can be found at JCI Insight.
Related Publications
- Cardiac Cytoarchitecture: How to Maintain a Working Heart-Waste Disposal and Recycling in Cardiomyocytes. Jordan Blondelle & Stephan Lange. Book Chapter in Cardiac Cytoarchitecture. Edited by Elisabeth Ehler. Springer International Publishing Switzerland. 2015. ISBN: 978-3-319-15262-2 (Print) 978-3-319-15263-9 (Online). Online Version
- Cullin E3 ligase activity is required for myoblast differentiation. Jordan Blondelle, Paige Shapiro, Andrea A. Domenighetti, Stephan Lange. JMB. 2017. dx.doi.org/10.1016/j.jmb.2017.02.012
- Cullin-3 dependent deregulation of ACTN1 represents a new pathogenic mechanism in nemaline myopathy. Blondelle J, Tallapaka K, Seto JT, Ghassemian M, Clark M, Laitila JM, Bournazos A, Singer JD, Lange S. JCI Insight. 2019 Apr 16. doi.org/10.1172/jci.insight.125665
Future Directions
We are currently investigating whether E3-ligases of the cullin protein family play a greater role for muscle specific protein turnover than previously anticipated. Of particular interest is the possible role that faulty cullin-3 function and accumulation of its substrates play for the development of neuromuscular diseases and cardiomyopathies.
Collaborators on this project
- Dr. Jeffrey Singer at Portland State University, OR
- Dr. Jane Seto at Murdoch Children's Hospital, Australia
- Drs. Careina Wallgren-Pettersson & Katarina Pelin at the University of Helsinki, Finland
Keywords: degradation, cullin, E3-ligase, ubiquitin.
|