August 22, 2013
See also earlier entry 07/30/13
|
On antibodies - case III
A couple of months back we started looking into phosphorylation levels of HDAC proteins for one of our projects. HDAC's (or Histone Deacetylases) are enzymes that remove acetyl-moieties from lysine residues of posttranslationally modified proteins. And, unlike the name suggests, HDAC's are responsible to deacetylate a whole load more substrate proteins than just histones. Either way, it is generally thought that HDAC activity is reflected through their phosphorylation state, with low phosphorylation levels on certain serine/threonine residues within the HDAC protein equating to low HDAC activity, and hence increased cellular protein acetylation levels.
(I know that this is a 'laymans' description of what HDAC's do and how their activity is regulated. It's a whole lot more complicated.)
During the course of our investigations we came up with the hypothesis that a certain protein kinase C isoform may be responsible for the phosphorylation of certain HDAC's, and hence for the regulation of HDAC activity. There are a few 'good' antibodies out there, and Cell Signaling Technologies is offering two nice phospho-specific HDAC4/5/7 antibodies, one of which is this one.

So we tested if PKC is able to phosphorylate part of the HDAC5 and HDAC7 proteins that contain the phosphorylation sites recognized by the antibody through in vitro kinase assays. In addition, we mutated the specific series to alanines, to see if there are more serines/threonines within this part of HDAC5 or HDAC7 that can be phosphorylated by PKC. The assay worked brilliantly using radioactively labeled ATP, demonstrating that PKC may indeed be able to phosphorylate HDAC5 and HDAC7. Moreover, serine-alanine phospho-mutants showed a lower level of phosphorylation, indicating that S498 of HDAC5, and S486 of HDAC7 are targets for PKC activity, and that additional phosphorylation sites may be present within the tested HDAC protein fragments.
Now, because we always try to validate antibodies (after encountering a widely published "phospho"-specific antibody of phospholamban that was NOT phospho-specific at all), our kinase assay with the wildtype and mutant HDAC fragments is an ideal way to evaluate the specificity of this antibody. So we repeated the kinase assay using unlabeled ATP and either wildtype or serine-alanine mutant for HDAC7. The results turned out to be somewhat troublesome.
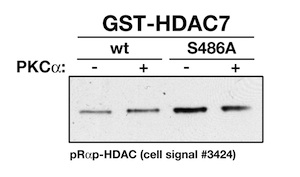
So I went ahead and contacted the tech support of Cell Signaling with this result:
From: Stephan Lange
To: Cell Signaling Tech Support
Hello,
I have a question regarding pHDAC antibody #3424.
[. . .] the result I got indicates that the antibody detects phosphorylated as well as unphosphorylated HDAC7, and it even detects HDAC7 that has been mutated at residue S486 (to alanine)? How is this possible? Did we get a bad lot (lot# 3; ref:07/2012) of the antibody?
Thank you for your help.
Best
Stephan
|
To which Cell Signaling replied:
Thank you for contacting CST Technical Support with your recent #3424 Phospho-HDAC4 (Ser632)/HDAC5 (Ser498)/HDAC7 (Ser486) Antibody data. I have not observed non-phospho cross-reactivity with the antibody. I visualize a depletion of signal in whole cell extracts treated with lambda phosphatase. I have not tested the antibody with any site mutations.
However, I will be testing this antibody with peptides generated to neighboring phospho-sites (S487), other phospho-sites with sequence similarities (S358), and alanine mutations to help clarify the results you received.
|
Simply phenomenal! This is great tech support! So after waiting a couple of weeks Cell Signaling got back to me with the results:
We generated over 30 peptides to test specificity, which took longer than anticipated. I tested cross-reactivity with the following serine residues.
HDAC4: Ser246, Ser467 and Ser632
HDAC5: Ser259, Ser498 and Ser661
HDAC7: Ser155, Ser358 and Ser486
I tested alanine substitutions for all serine residues and dual phospho-peptides for Ser498/499, Ser632/633, Ser661/662 and Ser486/487. I tested peptide with both of our phospho-HDAC antibodies, #3424 Phospho-HDAC4 (Ser632)/HDAC5 (Ser498)/HDAC7 (Ser486) Antibody and #3443 Phospho-HDAC4 (Ser246)/HDAC5 (Ser259)/HDAC7 (Ser155) (D27B5) Rabbit mAb. I found that #3424 cross-reacts with phosphorylated Ser467 and Ser661 in addition to Ser632, Ser498 and Ser486. I did not find any additional cross-reactivity with #3443 besides Ser246, Ser259 and Ser155. Neither antibody cross-reacts with peptides with alanine substitutions. I will be updating our #3424 web page and datasheet to reflect these findings.
|
Well - there you have it. It is somewhat phospho-specific, but sure enough its not just the sites advertised.
Either way. Cell Signaling handled the whole thing very well, invested a lot of work to further characterize their product. I lift my hat in respect!
|