Lab Blog
 In the works |
 Analysis |
 Images |
 Science |
 Experiments |
 Organization |
JULY 30, 2013
Upadted 08/10/13 Updated 09/03/13 |
On antibodies - cases I & IIHowever, it starts to look really bleak once you start 'looking behind the curtain' to verify some of the antibodies for specificity. In the following I am going to give two examples of what you can find outright - and what only becomes apparent when you do some experiments. Case 1For the first example we don't have to look very far: Santa Cruz Biotech.Don't get me wrong. Santa Cruz has some awesome antibodies, but also some abysmal failures. At least you get 1ml of antibody for the same price as 50ul at other companies (with approximately the same concentration). So you get at least a good amount for your money. But just looking at the homepage with its sample images for antibodies reveals some shady things. Here is a really bad example: sc-16720 and sc-16718, both supposed to be phospho-specific antibodies against beta2-AR. One recognizes beta2-AR phosphorylated at 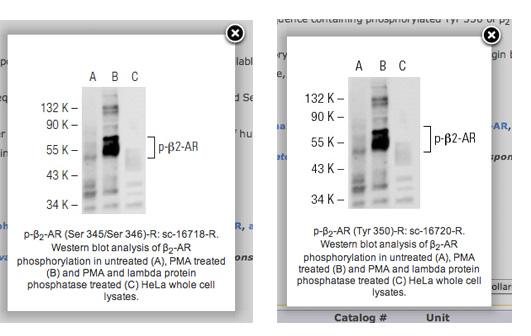 Hmmm. These look somewhat similar. No wait, they look IDENTICAL. Well, at least they took the time to edit the figure legend appropriately. Either way - that is not to say that the actual antibodies don't work. Matter of fact they do seem to work in immunofluorescence for me (but it needs more testing).
Case 2There are some proteins in the heart whose functions have been intensely studied over the years. One of these is the small phospholamban protein (PLN, PLB), which plays a pivotal role for the regulation of calcium uptake into the SR through its interaction with the ATP driven calcium pump SERCA. Now PLN can be phosphorylated at three different sites: S10, S16 and T17, with S16 being the best characterized. Phosphorylation levels of PLN have been shown in many publications to be deregulated in disease, with huge implications for molecular mechanisms of disease etiology.Now phospho-specific PLN antibodies can be ordered from several companies. A typical datasheet entry for the immunogen of a phospho-PLN antibody looks like this:  So they used a synthetic peptide that is phosphorylated at one specific amino acid as antigen, and then the antibody serum gets purified through affinity purification, typically by protein G or protein A beads. So far so good. Until you try to verify the antibody. We happened to work a little bit on PLN, expressing it recombinantly in bacteria and purifying it. And we did that for the wildtype variant, and for a mutant that cannot be longer phosphorylated at a specific residue. You see where this is going? So we used our pure PLN proteins, phosphorylated them in vitro and tested the antibodies. 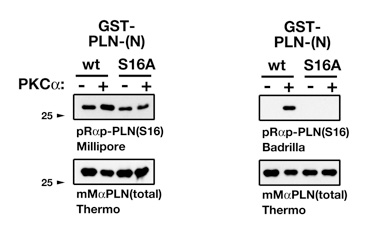 On the top left you see a "bad" 'phospho-specific' antibody that supposedly recognizes Ser16-PLN, and on the top right is a truly specific Ser16-PLN antibody as a comparison. So I contacted Millipore to point out the problem we encountered. Here is the first encounter with the tech support (which was actually quite good):
My reply to this must have been somewhat convincing, as they offered me to send another batch of the same antibody. However the new batch gave exactly the same result. Simply put the two batches of the antibody are NOT phospho specific! In the end tech support apologized for the problems. However the antibody is still sold from the company, and - also quite worryingly - the results obtained by this antibody are published. The problem here in my view is the peptide synthesis and purification processes. Did anyone do a quality control of the synthesized peptide? Also, purifying the antibody from sera with protein-A or protein-G is good. Better would have been to purify against the epitope - the phosphorylated peptide used for immunization. So these are just two examples I encountered in the last months. There are many more bad examples; maybe something for another blog entry sometime in the future. Fact is though that you cannot truly trust any antibody until you have tested them. Simplest test (although with its pitfalls) is overexpressing a tagged version of your protein of interest and check if you can see a (additional) band in a western blot or higher protein levels in immunofluorescence. Another (maybe the best, though in most cases rather challenging) control is to test an antibody on samples of the knockout animal/tissue/cells to see if a specific band disappears. All these problems aside. Antibodies are an ever more important staple to investigate proteins. And there is not really a way, then keep using them - as long as they truly recognize what they are supposed to be made against. |
| Share this blogpost Tweet |
|
Please remember the disclaimer. |
Section 'Sub' Navigation: